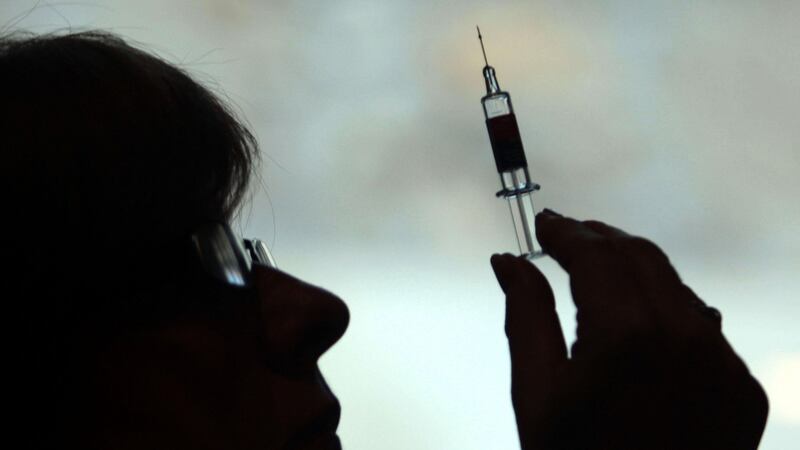
Patients infected with HIV could soon be offered a long-lasting injection to control the virus and keep Aids at bay.
Results from a pivotal Phase III trial show the monthly jab is as effective as the standard current treatment that involves taking a daily cocktail of pills.
A Phase III trial is the final testing hurdle before a new drug can be licensed.
Replacing a daily regimen of multiple pills with a single injection is expected to improve compliance and ensure more patients get the treatment they need.
As a result, the jab could also help to reduce the spread of HIV.
Currently most people infected with HIV take a combination of three or more pills per day to prevent the virus replicating and triggering full-blown Aids.
The new jab contains two anti-retroviral drugs, cabotegravir and rilpirivine. At present it has to be administered by a nurse or doctor, though future versions could be self-injected.
The ATLAS trial involved 618 patients from 13 countries who were given a three-pill standard treatment before switching to the monthly injection.
Newly announced headline results show the trial met its primary “endpoint”, or goal, by demonstrating that the jab matched the standard treatment for effectiveness over a period of 48 weeks.
Dr John Pottage, chief scientific and medical officer at ViiV Healthcare, the specialist pharmaceutical company running the trial, said: “This novel approach is another step towards potentially reducing the treatment burden for people living with HIV.
“The data from ATLAS suggest a long-acting injectable 2DR (two-drug regimen) of cabotegravir and rilpivirine may offer an alternative to daily, oral three-drug therapy for people who have previously achieved viral suppression.
“If approved, this regimen would give people living with HIV one month between each dose of anti-retroviral therapy, changing HIV treatment from 365 dosing days per year to just 12.”
ViiV Healthcare is owned by the drug companies GlaxoSmithKline, Pfizer and Shionogi and specialises in HIV treatment.
It is developing the HIV jab in collaboration with Janssen Sciences Ireland, part of the Johnson & Johnson family of drug and consumer product companies.